Non-Invasive Prenatal Testing (NIPT) – The SAFE Test
On this page:
What is the SAFE test?
The St George’s Antenatal Fetal Evaluation (SAFE) test is a Non Invasive Prenatal Testing service, screening for for Down’s, Edwards’ and Patau’s syndromes only, in line with UK National Screening Committee (UKNSC) recommendations. The SAFE test has been set up as a collaboration between the Fetal Medicine Unit and the St George’s Genomics Service, which is a UKAS accredited medical laboratory, No. 8127
NHSE Referrals
The SAFE test is offering non-invasive prenatal testing (NIPT) within the Fetal Anomaly Screening Programme (FASP) and via the National Genomics Testing Directory, referral code R445. All NHSE funded referrals can only be made via allocated NHS referring hospitals. Please contact your local antenatal screening team for more information.
For more information on access and eligibility for NIPT via FASP referrals see Screening tests for you and your baby If you have received a higher chance combined or quadruple test result, please refer to your choices after a higher chance screening result.
For more information on access and eligibility for NIPT via the R445 referral pathway please refer to the National Genomic Test Directory https://www.england.nhs.uk/wp-content/uploads/2018/08/Rare-and-inherited-disease-eligibility-criteria-version-6-January-2024.pdf
For patients accessing the SAFE test outside of these national pathways please continue to access the information available on this website.
What is NIPT?
Non-Invasive Prenatal Testing (NIPT) is a blood test taken from the mother in pregnancy, that uses cutting edge DNA technology to evaluate whether a baby has a high chance of a certain chromosomal condition.
How does the SAFE test work?
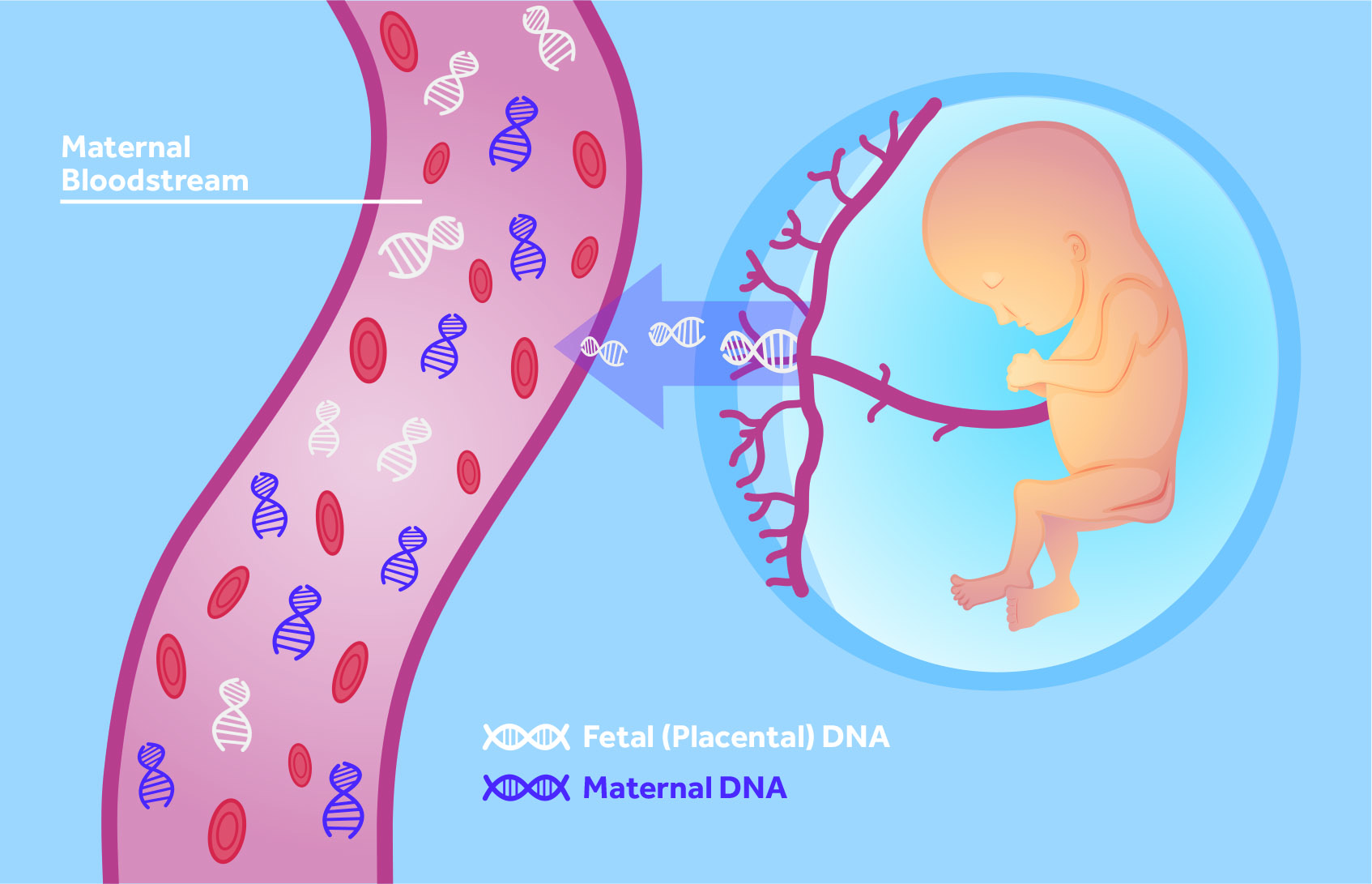
During pregnancy the placenta sheds cell free DNA (cfDNA) into the mother’s bloodstream. As a result, the mother’s blood contains a mixture of placental and maternal cfDNA. By evaluating the cfDNA in the blood and combining this with the mother’s background chance of a trisomy (mother’s age or the combined test results offered within the NHS), a likelihood ratio is obtained to predict whether or not the baby is more likely to have a chromosomal condition such as Down’s syndrome.
Why have the SAFE test?
As part of the national screening pathway women are offered the ‘combined test’ in the first trimester of pregnancy. This evaluates hormonal blood levels with ultrasound findings to assess the chance of chromosomal or structural anomalies. Although the range of conditions that can be detected by this method is broader than the number of conditions identified by the SAFE test, the SAFE test has a higher detection rate for Down’s, Patau’s and Edward’s syndrome.
In addition if the ‘combined test’ result is available, the SAFE test is able to incorporate this to provide a more individualised result.
What is Down’s syndrome?
People with Down’s syndrome (or Trisomy 21) have an extra copy of chromosome 21 (three chromosome copies rather than the usual two). Around one in every 1000 babies born in the UK will have Down’s syndrome and there are over 40,000 people in the UK with the condition. Anyone can have a baby with Down’s syndrome, although the chance increases for older mothers, most babies with Down’s syndrome are born to younger women.
Down’s syndrome is a life-long condition typically associated with some level of learning disability. Some health conditions are more common in people with Down’s syndrome, although most medical issues can be treated. Downs’ syndrome is extremely variable and it is impossible to know what life will be like for you and your baby. Some children and adults will need long term support, however many young people attend mainstream schools and live fairly independent lives with varying degrees of assistance.
Can the SAFE test screen for all conditions?
No-Down’s syndrome is the most common condition looked for as well as two rarer and conditions known as Edwards’ syndrome (trisomy 18) and Patau’s syndrome (trisomy 13).
What are Edwards’ and Patau’s syndrome?
Edwards’ and Patau’s syndromes are life limiting conditions and will cause a wide variety of developmental and health difficulties, some of which can be very serious. Around 70% of pregnancies affected by Edwards’ and Patau’s syndrome will end in miscarriage or stillbirth. Partial forms of Edwards’ and Patau’s syndrome have a lesser impact upon the child.
Who can have the SAFE test?
The test is suitable from 10 weeks of pregnancy for all single and identical twins pregnancies, including IVF, egg donor or surrogate pregnancies. For non-identical and vanishing twin, test sensitivity is reduced.
The test is not suitable for multiple pregnancies (greater than twins), if the mother has cancer, a chromosomal or genetic condition (including Down’s syndrome). It is also unsuitable for mothers who have undergone a blood transfusion in the last 4 months, had transplant surgery, immunotherapy or stem cell therapy.
Clinical Performance
Clinical performance of the SAFE/IONA®test
Sensitivity Specificity Positive Predictive Value (PPV) Negative Predictive Value (NPV)
Trisomy 21 >99% >99% 99% >99%
Trisomy 18 97% >99% 97% >99%
Trisomy 13 >99% >99% 97% >99%
Observed performances are based on Post-Market Surveillance of the IONA® Nx workflow in 76,989 singleton and monochorionic twin pregnancies, from a population of women who are predominantly at a higher risk of having a fetus with Down’s syndrome (see prevalence for the population tested). Performances are dependant of laboratories fully reporting discordant results to Yourgene Health as they occur. From data held on file by Yourgene Health. Correct as of 31st Oct 2023.
The SAFE test uses the IONA® Nx workflow supplied by Yourgene Health. For information on current clinical performance, visit Yourgene Health – IONA Nx Clinical Performance
NOTE: The SAFE test only offers screening for Trisomy 21, 18 and 13 therefore any other statistics shown on the Yourgene website do not apply to the SAFE test.
DEFINITIONS:
Sensitivity – the proportion of people/babies confirmed to have a condition that is reported as higher chance from an NIPT screening test
Specificity – the proportion of people/babies confirmed not to have a conditionthat is reported as lower chance from an NIPT screening test
Detection rate – the proportion of truly affected pregnancies that are reported as high chance.
Positive predictive value (PPV) – this is the number of truly positive cases that have been identified out of all the cases that have been reported as screen positive/high chance.
Negative predictive value (NPV) – this is the number of truly negative cases that have been identified out of all the cases that have been reported as screen negative/low chance
How is the SAFE test reported?
Low chance: means that it is very unlikely that your pregnancy is affected by trisomy 21, 18 or 13, and therefore very unlikely that your baby has Down’s, Edwards’ or Patau’s syndrome.
High chance: means that there is an increased chance that your baby will have trisomy 21, 18 or 13 and that the result should be confirmed by an invasive diagnostic test.
No call result: in a very small number of cases (1 in 200) tests may not yield a result for a variety of reasons. In this instance the clinical team will discuss your available options.
What happens if I get a “high chance” result?
If the SAFE test result shows a high chance of a chromosomal condition you will be offered an invasive diagnostic test such as amniocentesis or chorionic villus sampling (CVS). These tests give a definite ‘yes’ or ‘no’ result as to whether your baby has Down’s, Edwards’ or Patau’s syndrome. Both procedures involve using a fine needle to collect either a small sample of the amniotic fluid that surrounds the baby (amniocentesis) or a small sample of cells from the placenta (CVS).Although these invasive procedures give a definitive diagnosis they do carry a small risk of miscarriage. The chance of miscarriage is often a dilemma for parents, with many women opting to have NIPT, such as the SAFE test before proceeding to an invasive procedure.
It is important to remember that NIPT is a screening test which means that occasionally false positive and false negative results do occur. It is a good idea to consider the need to be certain about the diagnosis compared to the risk of miscarriage associated with the invasive procedure. Some women, who would continue their pregnancy anyway, may be happy to proceed without invasive testing. An invasive test would be needed to confirm the SAFE test result for those considering termination of pregnancy.
Your midwife and/or obstetrician will be on hand to answer any questions you may have and to support you through this time.
Who can I contact for further information?
If you have any questions about the SAFE test, please contact your named midwife or consultant obstetrician. To contact directly, please email: theSAFEtest@stgeorges.nhs.uk
Referring Clinics
Please note samples can only be referred from clinics or NHS hospitals that have been registered with the SAFE test laboratory. If you are interested in referring samples for testing, please contact the laboratory directly to discuss.
Registered clinics/hospitals will be provided with information regarding sample collection, transport and reporting.
Complaints and Confidentiality
Please refer to the main St George’s Genomics Service pages for information on processes for handling of complaints and patient information.
Other useful organisations
Down’s Syndrome Association
Tel: 020 8682 4001
Positive about Down syndrome
Tel: 07814 929306
Support Organisation for Trisomy 13/18 (SOFT) UK
Tel: 03300881384
Antenatal Results and Choices (ARC)
Tel: 020 7631 0285
The SAFE test is a CE-marked in vitro diagnostic test from Yourgene Health plc (The IONA®Nx test). Yourgene Health plc is a UK based molecular diagnostics company working in partnership with St George’s University NHS Hospitals Foundation Trust to create a UK Centre of Excellence in bringing the first regulated NIPT test to more pregnant women.





