St George’s one of the first to offer new lifesaving covid-19 treatment
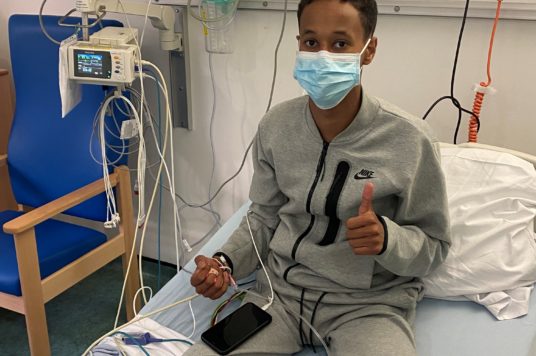
A 17-year-old critically ill covid patient and TikTok star was one of the first in the UK to receive a ground-breaking antibody treatment at St George’s Hospital approved for use just a few days ago.
Max Khadar was the first to be offered the new lifesaving drug, Ronapreve, at St George’s today (Friday 24 September).
Max was born with a heart condition which means he is immunosuppressed, puts him in the high-risk category for covid-19 and is unable to be vaccinated.
Max said: “I feel very thankful, very grateful and, actually, very special to be the first at St George’s and one the first in the UK to receive this treatment.
He added: “I’ve been looked after by the NHS ever since I was born due to my condition, so I’d like to say thank you to all NHS staff for caring for people like me who are vulnerable and need that additional support, particularly at the moment with covid-19.”
Ronapreve is made up of two monoclonal antibodies and will be offered to vulnerable patients, like Max, who test positive for covid-19 but are unable to build their own immune response to fight the disease due to being immunosuppressed.
The treatment was approved following results from the RECOVERY trial which St George’s recruited patients onto. Evidence from the trial showed the treatment reduced hospital stays by four days and risk of death by a fifth.
St George’s Chief Medical Officer, Dr Richard Jennings, said: “Ronapreve reduces the risk of dying for older patients and those who are more vulnerable – and, importantly, it also speeds up recovery time.
“We are very proud to be one of the first hospitals in the UK to roll out this new form of treatment, after having undertaken the RECOVERY trial at St George’s too.”
Since the beginning of the pandemic, St George’s and St George’s, University of London have been leaders in amongst covid-19 research, having undertaken 60 covid-19 studies and recruited 7019 participants into trials.
Dr Tihana Bicanic, Reader and Consultant in Infectious Diseases at St George’s, University of London and St George’s Hospital and Principal Investigator of the RECOVERY trial, said: “At St George’s, we are extremely proud of our participation in the RECOVERY trial, having enrolled a total of 275 patients since March 2020. RECOVERY has repeatedly shaped and defined how we treat COVID-19 globally, leading to lives saved following positive results for dexamethasone and tocilizumab and most recently Ronapreve. We are very excited to now be rolling out Ronapreve to our most vulnerable NHS patients who have been unable to mount an immune response to the virus.
Dr Carolyn Johnston, Consultant Anaesthetist and Deputy Chief Medical Officer for Improvement, said: “Many of our clinicians have recruited patients to the clinical trials all though the pandemic, so to see this drug becoming available to help sick and vulnerable patients and save lives in London from today is especially pleasing.”
Dr Daniel Forton, Consultant Hepatologist and Associate Medical Director for Research, said: “St George’s and St George’s, University of London have worked very hard to conduct one of the broadest ranges of research studies and clinical trials nationally. Major advances such as the use of this new treatment come about because we have been successful in embedding research in everyday NHS practice and through the hard work of our research nurses, doctors and the commitment from our patients.”
Ronapreve is the first neutralising antibody medicine specifically designed to treat covid-19 to be authorised by the Medicines and Healthcare products Regulatory Agency (MHRA) for use in the UK. The drug is administered via a drip and works by binding to the virus’ spike protein, stopping it from being able to infect the body’s cells.
Thousands of patients across the UK will benefit from this treatment once fully rolled out across the UK but it doesn’t reduce the need for members of the public to be vaccinated and St George’s continues to offer the jab to staff and patients.
Notes to editors
Additional information
- St George’s has opened 60 covid-19 studies, recruited to 45 and recruited 7019 participants
- St George’s is ranked joint third in the UK for the number of Urgent PHE studies recruited to – 29 studies
- St George’s has a total of nine vaccine studies with a total of 1277 recruits and seven therapeutic studies with 439 recruits.
For more information or photos, please contact philippa.harper@stgeorges.nhs.uk.